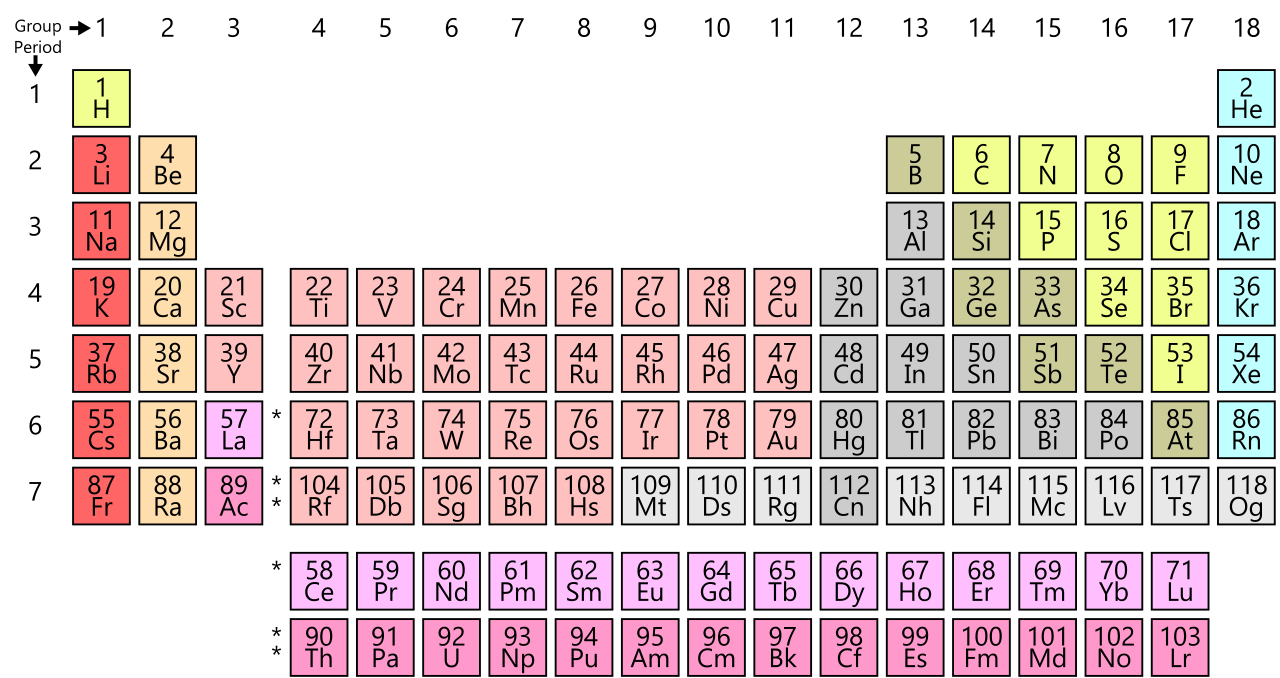
Periodic Table of Chemical Elements
(Image Sources: Wikipedia.org, By Offnfopt - Own work, Public Domain, https://commons.wikimedia.org/w/index.php?curid=62296883)
By Glenn A. Walsh
Reporting for SpaceWatchtower
All ordinary matter in the Universe
(not including “Dark Matter”) is composed of chemical elements.
It was not until 150 years ago today (on 1869 February 17) that a
logical classification system was developed for chemical elements:
The Periodic Table of Chemical Elements.
While British, French, and German
scientists had earlier attempted to develop systems to organize the
then-known elements, it was a Russian professor of chemistry, Dimitri
Ivanovich Mendeleev, who created this first comprehensive
representation of elemental reality, which classified the then-known
63 elements in order of atomic weight.
And, unlike earlier systems, Professor
Mendeleev did something unprecedented with his Table. He left spaces
in his Table for elements, with predicted atomic masses and chemical
properties, which had yet to be discovered! The development of the
Periodic Table of Chemical Elements is an interesting story, showing
the eccentricity and obsession of Professor Mendeleev.
After conducting research in Europe, in
1861 Dimitri Mendeleev returned to Russia and later began teaching
chemistry at the Saint Petersburg Technical Institute. Realizing that
there was no current textbook on organic chemistry, he wrote his
first book, Organic Chemistry, in 1861. This was considered
one of the most authoritative books on the subject in the middle of
the 19th century.
Although only 27 years-old at the time,
he had a long flowing beard and disheveled hair (only cut once a
year), looking much like the stereotypical, eccentric scientist, long
before Albert Einstein made the look popular. But, it is reported
that he was popular with his students.
Despite his new textbook, Professor
Mendeleev continued to be concerned that, without an adequate
chemistry classification system, his students would continue having
trouble understanding the subject. As reported in the 2000 book
Mendeleyev’s Dream: The
Quest for the Elements by
Paul Strathern, Professor Mendeleev wrote:
The
edifice of science requires not only material, but also a plan, and
necessitates the work of
preparing the materials, putting them
together, working out the plans and symmetrical
proportions of the
various parts.
According
to a Khan Academy on-line course article, Professor Mendeleev
actually developed his first Periodic Table from a dream he had experienced!
In
1867, Professor Mendeleev started writing a second chemistry book
concentrating on inorganic chemistry. This book, Principles
of Chemistry (in
two volumes), also became a standard text for the field, for several
decades.
With
the writing of this book, he started concentrating on finding a way
to classify elements. While he tried to use the two organizing
systems common at that time, organizing by atomic mass or by chemical
properties, he found neither way as satisfying. Then, he hit on a new
system which combined both original systems into a single framework.
This
single framework was his breakthrough. And, it seems this system was
inspired by the card-game “Solitaire”! Solitaire
arranges game-cards both by suit, horizontally, and by number,
vertically.
Professor
Mendeleev created 63 cards, one for each of the known elements at the
time. Then, he started rearranging the cards by atomic mass and by
chemical properties. He spent a great deal of time, wherever he was,
organizing and re-organizing the 63 cards.
On
1869 February 17, he again started rearranging the cards after
breakfast, and before he had to leave to catch a train. Well, he
completely forgot about catching the train, and continued working on
the project for three days. After testing-out many different
sequences for the cards, he suddenly realized that there were gaps in
the order of atomic mass.
According
to the 2000 book Mendeleyev’s
Dream: The Quest for the Elements by
Paul Strathern, Professor Mendeleev fell asleep after the three-day
effort. Upon awakening, he declared, “I saw in a dream, a table,
where all the elements fell into place as required. Awakening, I
immediately wrote it down on a piece of paper.”
He
called his discovery the Periodic Table of the Elements. This was due
to his discovery of the “Periodic Law,” as he found that when the
elements were arranged in order of increasing atomic mass, elements
with similar chemical properties recurred at regular intervals, or
periodically, on his chart.
Actually,
his original Table did not completely use atomic mass as the
organizing principle; there were exceptions. Professor Mendeleev did
not realize it, but he had actually organized this Table by “Atomic
Number,” which is the number of positively-charged protons in the
atom (and negatively-charged electrons which orbit the atom).
This
periodicity, of the elements listed by ascending Atomic Number, comes
directly from the periodic repeating of similar electron
configurations in the outer shells of their respective atoms.
Professor Mendeleev went further by
using the patterns he found in his Table to predict the properties of
elements which had not yet been discovered. He left blank spots as
place-holders in his Table, for the missing elements for which he was
predicting their existence.
Professor Mendeleev's first Periodic
Table of the Elements was presented to, and published by, the Russian
Chemical Society on 1869 March 6. He continued working on the Table,
publishing improved Tables, including one in 1871.
Professor Mendeleev's Periodic Table of
the Elements was not immediately accepted by other scientists.
However within 20 years, three of the “missing” elements which
his Table predicted were discovered: Gallium (1875), Scandium (1879),
and Germanium (1886); and they all included the basic chemical
characteristics that Professor Mendeleev had predicted. The Periodic
Table of the Elements then began to be accepted by the scientific
community.
As with much of science, several
scientists were also developing the idea of organizing the elements
in some way. In 1787, the first list of the then-known 33 elements
was produced by French chemist Antoine Lavoisier, working with
Antoine Fourcroy, Louis-Bernard Guyton de Morveau and Claude-Louis
Berthollet. In 1817, German chemist Johann Döbereiner noticed that,
when the elements' properties are considered, they could be placed in
groups of three. In 1857, French chemist Jean-Baptiste-André Dumas
tried to organize the elements, mathematically, based on atomic
weight.
On 1863 February 7, British chemist
John Newlands published a Table of the Elements. He also found that there
was periodicity in the atomic mass of the elements and their chemical
properties. However, John Newlands' Table of the Elements was not well
received by the scientific community, and he did not pursue further
research in the area. Although his Table did not accurately predict
the characteristics of future to-be-discovered elements, he may be
the first person to recognize periodicity among the elements, even though he had trouble
clearly identifying it.
In 1870, German chemist Julius Lothar
Meyer published a paper describing a Table of the Elements similar to the
one described by Professor Mendeleev, but a year later. It was
probably Professor Mendeleev's confidence in the “place-holder”
elements' predicted properties that made his Periodic Table of
the Elements the most accepted.
National Periodic Table Day is
celebrated each year on February 7, as English analytical chemist
John Newlands published his Table of the Elements on 1863 February 7.
This unofficial, national holiday was created and publicized by a
chemistry teacher in the Jefferson County Public Schools in Kentucky,
David T. Steineker.
An interesting coincidence is that Dimitri Mendeleev's 1834 birth-date, as dated in the Western Hemisphere, was also February 7. At his birth-home in western Siberia, the date would have been February 8 as determined by the Gregorian Calendar. However, at this time Russia was still using the Julian Calendar (known as Old System or O.S.); by the Old System his birth-date was recognized as January 27.
An interesting coincidence is that Dimitri Mendeleev's 1834 birth-date, as dated in the Western Hemisphere, was also February 7. At his birth-home in western Siberia, the date would have been February 8 as determined by the Gregorian Calendar. However, at this time Russia was still using the Julian Calendar (known as Old System or O.S.); by the Old System his birth-date was recognized as January 27.
And, due to this year's 150th
anniversary of the Periodic Table of Chemical Elements, on 2017
December 20 the United Nations General Assembly proclaimed the year
2019 as the International Year of the Periodic Table of Chemical
Elements.
Internet Links to Additional Information ---
Periodic Table of Chemical Elements: Link >>> https://en.wikipedia.org/wiki/Periodic_table
Periodic Law: Link >>> https://en.wikipedia.org/wiki/Periodic_trends#Periodic_law
Dmitri Ivanovich Mendeleev:
Link 1 >>> http://www.chem.msu.su/eng/misc/mendeleev/welcome.html
Link 2 >>> https://en.wikipedia.org/wiki/Dmitri_Mendeleev
Khan Academy. "Periodic Table of Elements." Crash Course Chemistry On-Line Student Course.
Link >>> https://www.khanacademy.org/partner-content/big-history-project/stars-and-elements/knowing-stars-elements/v/bhp-periodic-table-crashcourse
Khan Academy. "Dmitri Mendeleev." Crash Course Chemistry On-Line Student Course.
Link >>> https://www.khanacademy.org/partner-content/big-history-project/stars-and-elements/knowing-stars-elements/a/dmitri-mendeleev
Scerri, Eric R. "The Evolution of the Periodic System."
Scientific American 2011 Jan. 21.
Link >>> https://www.scientificamerican.com/article/the-evolution-of-the-periodic-system/
Periodic Table Day: Link >>> http://www.periodictableday.org/
2019: International Year of the Periodic Table of Chemical Elements:
Link >>> https://iupac.org/united-nations-proclaims-international-year-periodic-table-chemical-elements/
Source: Glenn A. Walsh Reporting for SpaceWatchtower, a project of Friends of the Zeiss.
Sunday, 2019 February 17.
Like This Post? Please Share!
More Astronomy & Science News - SpaceWatchtower Twitter Feed:
Link >>> https://twitter.com/spacewatchtower
Astronomy & Science Links: Link >>> http://buhlplanetarium.tripod.com/#sciencelinks
Want to receive SpaceWatchtower blog posts in your in-box ?
Send request to < spacewatchtower@planetarium.cc >.
gaw
Glenn A. Walsh - Informal Science Educator & Communicator:
< http://buhlplanetarium2.tripod.com/weblog/spacewatchtower/gaw/ >
Electronic Mail: < gawalsh@planetarium.cc >
Project Director, Friends of the Zeiss: < http://buhlplanetarium.tripod.com/fotz/ >
SpaceWatchtower Editor / Author: < http://spacewatchtower.blogspot.com/ >
Formerly Astronomical Observatory Coordinator & Planetarium Lecturer, original Buhl Planetarium & Institute of Popular Science (a.k.a. Buhl Science Center), Pittsburgh's science & technology museum from 1939 to 1991.
Formerly Trustee of the Andrew Carnegie Free Library and Music Hall, Pittsburgh suburb of Carnegie, Pennsylvania.
Author of History Web Sites on the Internet --
* Buhl Planetarium, Pittsburgh:
< http://www.planetarium.
* Adler Planetarium, Chicago:
< http://adlerplanetarium.
* Astronomer, Educator, Optician John A. Brashear:
< http://johnbrashear.tripod.com >
* Andrew Carnegie & Carnegie Libraries:
< http://www.andrewcarnegie.
